50 years ago, insulin pumps were contained in large backpacks—today, they can fit in your pocket. 40 years ago, robot-assisted surgery didn’t exist—today, robots are routinely employed for delicate operations such as heart surgeries, transplants, and ophthalmological surgeries. 10 years ago, pacemakers required complicated wiring and invasive surgery—today, leadless pacemakers can be implanted via catheter. 5 years ago, ways to improve your sight were limited to glasses, contacts, and laser eye surgery—today, you can get a cortical visual prosthesis implant.
Like cell phones, computers, autonomous vehicles, and other modern technologies, medical device technologies are evolving rapidly. Competitive medical device markets demand rapid product development to ensure first-to-market advantage. Products developed quickly may meet regulatory requirements, but still have undesirable anomalies.
Testing early in your product development process with simulation allows you to identify anomalies earlier (while they cost less to correct). Model-in-the-Loop (MIL), Software-in-the-Loop (SIL), and Hardware-in-the-Loop (HIL) simulation allow you to go beyond functional safety compliance to focus more on product quality and reliability, keeping your end users happy and healthy. In this blog post, we’ll be exploring the cost of poor quality, different kinds of product simulation, and how you can use simulation to expedite product development and improve quality.
The Cost of Poor Quality
The cost of correcting defects increases exponentially the further product development progresses. Changes in the design of a medical device require engineering effort, potentially new materials, updated documentation, further testing, and time. These changes can certainly cost you the benefits of being first-to-market , yet, it’s the costs that are incurred if an anomaly is discovered after a medical device is on the market that can be most detrimental: product recalls, brand reputation, or even human lives.
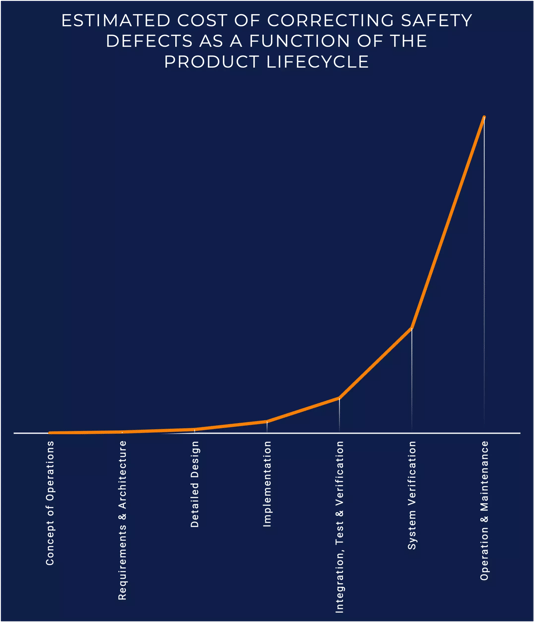
Estimated cost of correcting safety defects as a function of the product lifecycle
In the United States, the Food and Drug Administration (FDA) will take enforcement actions against a medical device manufacturer when a product is found to be in non-compliance with medical safety regulations. However, these enforcement actions focus exclusively on compliance and do little to improve medical device quality. The FDA has launched programs to encourage product quality, and safe and efficient manufacturing, but, ultimately, it is up to medical device manufacturers to improve product quality.
To improve product quality, medical devices should be tested early and often. Simulation via MIL, SIL, and HIL allow you to test your product before physical testing.
What are MIL, SIL, and HIL?
MIL, SIL, and HIL use representations of the different components of a controller and other similar electronic modules to simulate how the components will react in response to various stimuli and under various conditions. Each of these model-based testing methods are performed in a closed loop setting which provides greater accuracy (vs. open-loop testing) and allows for regression testing to ensure the controller functions as intended. Below is a brief definition of each.
Model-in-the-Loop Simulation
Model-in-the-loop (MIL) testing uses mathematical equations (i.e., models) that best represent the Plant (hardware) and the Controller in a simulated environment. During MIL simulation, the goal is to determine whether the Controller logic can control the Plant model as specified in the requirements. MIL simulation can be performed as early as during the system requirements definition phase of product development.
Software-in-the-Loop Simulation
Software-in-the-Loop (SIL) focuses on testing a Controller’s embedded software once it has been developed. SIL uses actual software code to mimic the software’s response to different inputs and outputs from the simulated Plant model. During SIL simulation, you’re looking to confirm that the Controller logic has accurately been translated into software code, while retaining the functionality confirmed during MIL simulation.
Hardware-in-the-Loop Simulation
Hardware-in-the-Loop (HIL) utilizes the physical Controller with the production intended embedded software installed. In a HIL test, the Controller is placed in a loop with a potential combination of the Plant model and real and simulated loads and sensors.

A Genuen HIL system
How to Use Simulation to Expedite Product Development and Improve Product Quality
It’s likely that you’re already using models early on in your product development during system requirements definition and high-level design. Using these models to simulate the functionality and responses of your products can help you accelerate your product design in a number of ways:
-
Identify Anomalies Early and Anticipate Defects Later
As mentioned previously, model-based simulation allows you to identify defects in a design early in your development lifecycle. This gives you the opportunity to correct them before your design progresses further. Additionally, model-based simulation tells you where potential issues may arise in the future. With this knowledge, you can more closely monitor these issues and adapt your testing plan to test them more rigorously. -
Reduce Development Time
It may seem like just another step slowing down your product development timeline, but model-based simulation can expedite your product development. By taking the time to perform simulation testing early in your design cycle, you can avoid potential defects later that will require time to resolve. And, if you do find flaws during simulation, you can typically fix them faster. If good quality doesn’t come at the cost of being first-to-market, it’s a win for both the consumer and the manufacturer. -
Reduce Development Costs
When defects in a product design are discovered later in the design process, they become more expensive to fix. The labor to redesign and rebuild a product, any new product materials, and the performance of further testing all increase your development costs. Discovering and resolving anomalies early means that there is less to re-do in your design.
(For even more advanced testing, you can combine your HIL testing with a physical test rig.)
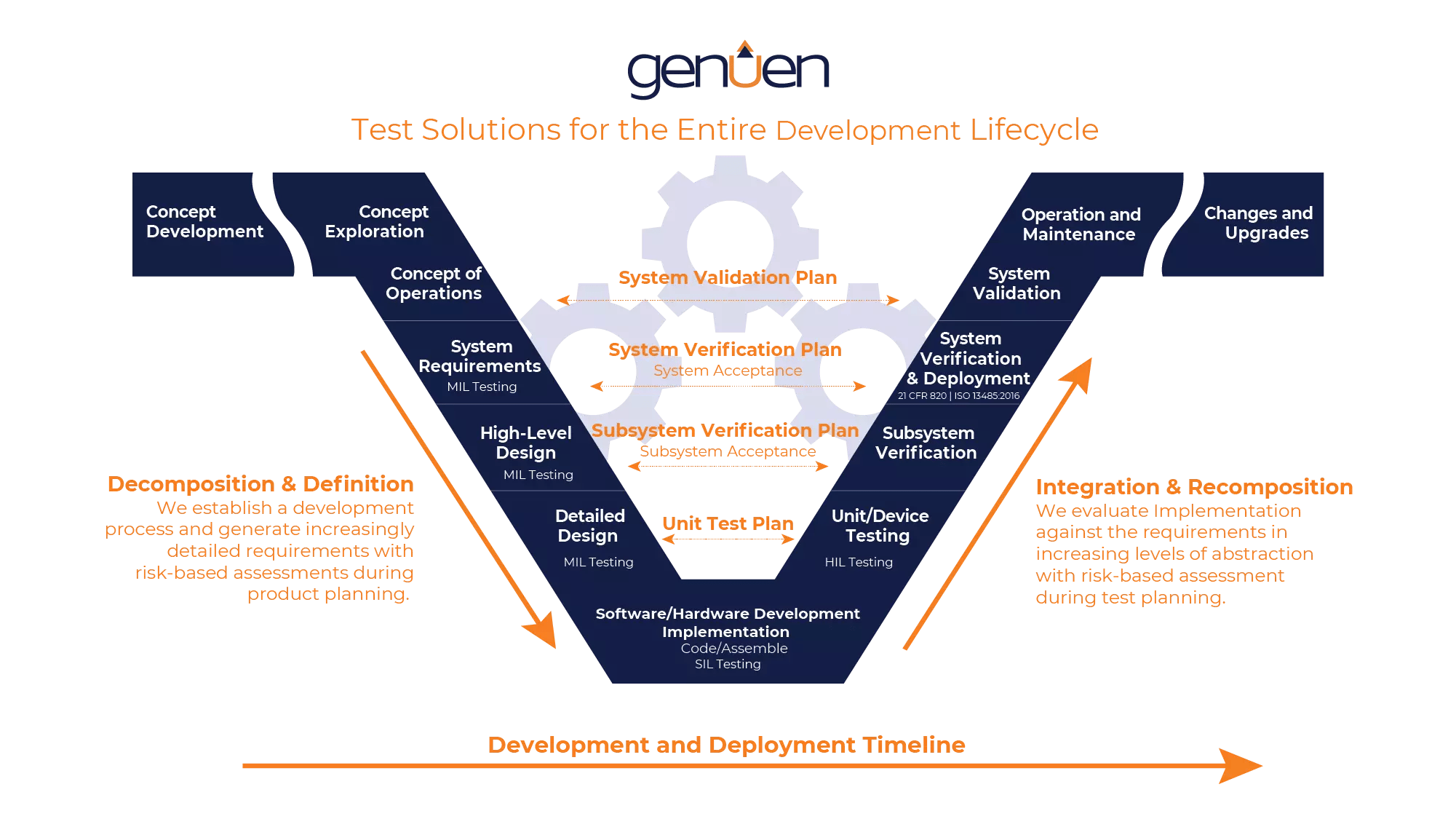
Genuen offers test solutions for every stage of the product development lifecycle
Application
HIL testers are largely based on open-standard software with COTS components. At Genuen, we’ve taken advantage of these to create platform-based HIL systems that reuse common elements previously developed for other testers. A universal, platform-based tester reduces your capital investment by allowing you to upgrade and add to an existing system when you’re ready to test other similar products. And, it takes less time to get your tester up and running. Faster development times and more robust data will lead to greater team productivity and cost savings. Your team can focus their efforts on building reliable and innovative products.
Perform Medical Device HIL Simulation with Genuen
Simulation enables you to produce better products for your end users, while maintaining business advantages, and even has the potential to reduce your overall development costs. At Genuen, we have the expertise to provide a cost-effective open-box HIL test system using open architectures, open standards, and COTS technology. Our flexible, scalable, user-friendly HIL systems are designed to grow with you.
To learn more about medical device testing throughout the entire product development cycle, download our ebook, "Life Cycle and Durability Testing for Medical Devices."




